THE STEAM
POWER CYCLE,
a brief overview.
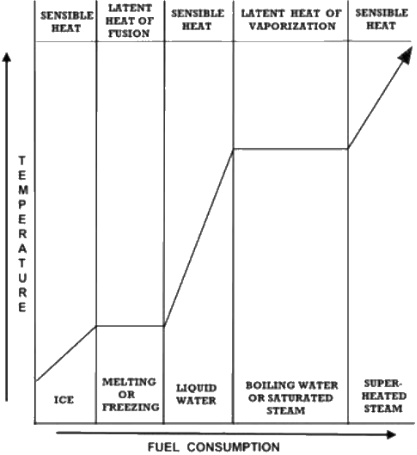
Water possesses three phases; solid, liquid and
gas. At most temperatures, adding heat to any of
these phases produces a proportional temperature
rise termed “sensible heat”. Sensible heat is heat
added to a process that is measured by a rise in
temperature or, as JP Joule put it, heat “indicated
by the thermometer”.


 GENERATION is the act of creating steam in boilers or steam generators.
Water isn’t always ‘sensible’ (That’s a joke, son). Under
normal sea level conditions, ice can’t exceed 32 degrees
F and water can’t pass 212 F even though heat is added.
Energy never disappears, it must go somewhere or do
something; ice uses the absorbed energy to change into
the water phase at 32F while water assumes the steam
phase at 212 F. The English word latent comes from
Latin latens, meaning “lying hid”. Temperatures that
remain constant until all the ice has melted or water
evaporated are thus referred to as “latent heat”.
GENERATION is the act of creating steam in boilers or steam generators.
Water isn’t always ‘sensible’ (That’s a joke, son). Under
normal sea level conditions, ice can’t exceed 32 degrees
F and water can’t pass 212 F even though heat is added.
Energy never disappears, it must go somewhere or do
something; ice uses the absorbed energy to change into
the water phase at 32F while water assumes the steam
phase at 212 F. The English word latent comes from
Latin latens, meaning “lying hid”. Temperatures that
remain constant until all the ice has melted or water
evaporated are thus referred to as “latent heat”.


 The above suggests:
* All sensible heat applied in superheating is available for conversion into work by the engine.
* All sensible heat applied to bring the water to the boiling temperature is lost.
* Part of the latent heat is available for conversion to work and part is lost to the cooling medium.
Limits to the Rankine Cycle can be understood by examining the roles latent and sensible heat play in the
four steam cycle elements:
GENERATION
* Adding sensible heat raises pressurized temperature to the boiling point.
* Adding latent heat transforms the water into pressurized steam.
* Adding more sensible heat raises the steam temperature, superheating it.
EXPANSION
* Superheat extracted from the steam in the engine does work.
* Latent heat extracted from the steam in the engine does work.
CONDENSATION
* Latent heat extracted from the exhaust steam, transforming it into condensate, does no work.
* Sensible heat extracted from the exhaust steam, cooling it below condensation temperature, does no
work
FEED
* Heat is neither added or extracted.
The amount of energy required to melt ice and
vaporize water are respectively called the latent heats
of fusion and vaporization. “Saturation temperature”
describes the temperature at the boiling point; at this
temperature steam can be saturated with (carry) an
unspecified amount of water of identical temperature.
“Superheated steam” describes the condition at which
steam has absorbed sensible heat beyond the latent
heat, warming the steam beyond saturation
temperature.
The above suggests:
* All sensible heat applied in superheating is available for conversion into work by the engine.
* All sensible heat applied to bring the water to the boiling temperature is lost.
* Part of the latent heat is available for conversion to work and part is lost to the cooling medium.
Limits to the Rankine Cycle can be understood by examining the roles latent and sensible heat play in the
four steam cycle elements:
GENERATION
* Adding sensible heat raises pressurized temperature to the boiling point.
* Adding latent heat transforms the water into pressurized steam.
* Adding more sensible heat raises the steam temperature, superheating it.
EXPANSION
* Superheat extracted from the steam in the engine does work.
* Latent heat extracted from the steam in the engine does work.
CONDENSATION
* Latent heat extracted from the exhaust steam, transforming it into condensate, does no work.
* Sensible heat extracted from the exhaust steam, cooling it below condensation temperature, does no
work
FEED
* Heat is neither added or extracted.
The amount of energy required to melt ice and
vaporize water are respectively called the latent heats
of fusion and vaporization. “Saturation temperature”
describes the temperature at the boiling point; at this
temperature steam can be saturated with (carry) an
unspecified amount of water of identical temperature.
“Superheated steam” describes the condition at which
steam has absorbed sensible heat beyond the latent
heat, warming the steam beyond saturation
temperature.


 GENERATION is the act of creating steam in boilers or steam generators.
Water isn’t always ‘sensible’ (That’s a joke, son). Under
normal sea level conditions, ice can’t exceed 32 degrees
F and water can’t pass 212 F even though heat is added.
Energy never disappears, it must go somewhere or do
something; ice uses the absorbed energy to change into
the water phase at 32F while water assumes the steam
phase at 212 F. The English word latent comes from
Latin latens, meaning “lying hid”. Temperatures that
remain constant until all the ice has melted or water
evaporated are thus referred to as “latent heat”.
GENERATION is the act of creating steam in boilers or steam generators.
Water isn’t always ‘sensible’ (That’s a joke, son). Under
normal sea level conditions, ice can’t exceed 32 degrees
F and water can’t pass 212 F even though heat is added.
Energy never disappears, it must go somewhere or do
something; ice uses the absorbed energy to change into
the water phase at 32F while water assumes the steam
phase at 212 F. The English word latent comes from
Latin latens, meaning “lying hid”. Temperatures that
remain constant until all the ice has melted or water
evaporated are thus referred to as “latent heat”.


 The above suggests:
* All sensible heat applied in superheating is available for conversion into work by the engine.
* All sensible heat applied to bring the water to the boiling temperature is lost.
* Part of the latent heat is available for conversion to work and part is lost to the cooling medium.
Limits to the Rankine Cycle can be understood by examining the roles latent and sensible heat play in the
four steam cycle elements:
GENERATION
* Adding sensible heat raises pressurized temperature to the boiling point.
* Adding latent heat transforms the water into pressurized steam.
* Adding more sensible heat raises the steam temperature, superheating it.
EXPANSION
* Superheat extracted from the steam in the engine does work.
* Latent heat extracted from the steam in the engine does work.
CONDENSATION
* Latent heat extracted from the exhaust steam, transforming it into condensate, does no work.
* Sensible heat extracted from the exhaust steam, cooling it below condensation temperature, does no
work
FEED
* Heat is neither added or extracted.
The amount of energy required to melt ice and
vaporize water are respectively called the latent heats
of fusion and vaporization. “Saturation temperature”
describes the temperature at the boiling point; at this
temperature steam can be saturated with (carry) an
unspecified amount of water of identical temperature.
“Superheated steam” describes the condition at which
steam has absorbed sensible heat beyond the latent
heat, warming the steam beyond saturation
temperature.
The above suggests:
* All sensible heat applied in superheating is available for conversion into work by the engine.
* All sensible heat applied to bring the water to the boiling temperature is lost.
* Part of the latent heat is available for conversion to work and part is lost to the cooling medium.
Limits to the Rankine Cycle can be understood by examining the roles latent and sensible heat play in the
four steam cycle elements:
GENERATION
* Adding sensible heat raises pressurized temperature to the boiling point.
* Adding latent heat transforms the water into pressurized steam.
* Adding more sensible heat raises the steam temperature, superheating it.
EXPANSION
* Superheat extracted from the steam in the engine does work.
* Latent heat extracted from the steam in the engine does work.
CONDENSATION
* Latent heat extracted from the exhaust steam, transforming it into condensate, does no work.
* Sensible heat extracted from the exhaust steam, cooling it below condensation temperature, does no
work
FEED
* Heat is neither added or extracted.
The amount of energy required to melt ice and
vaporize water are respectively called the latent heats
of fusion and vaporization. “Saturation temperature”
describes the temperature at the boiling point; at this
temperature steam can be saturated with (carry) an
unspecified amount of water of identical temperature.
“Superheated steam” describes the condition at which
steam has absorbed sensible heat beyond the latent
heat, warming the steam beyond saturation
temperature.